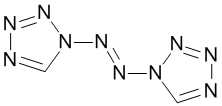
I’m sure Derek Lowe’s “Things I Won’t Work With” series is essential reading for most here (and for those that have missed out, take a look – my favorite was FOOF). He put one up a couple of years back on an interesting structure with 8 nitrogens in a row (two triazoles linked by 2 nitrogens). It was surprisingly stable, despite Derek’s title of that post. It also has a cool color effect where it changes color when you shine light on it (photochromism), due to the central double bond changing from trans to cis and back again.
My lab mates were talking about that and one pondered aloud what would happen if you used tetrazole instead? Luckily someone else has found out for us because, believe me, you didn’t want to be the one doing this particular piece of research.
Inevitably, it was Thomas M. Klapotke’s group that made it, published in 2011 in Inorganic Chemistry. Well, it was almost devoid of carbon by this point so why not? The graphical abstract tells the story eloquently: the structure with a back-drop of broken lab equipment. It was made in the exactly analogous fashion to the 8 nitrogen analog, but that is where the similarity ends. When the Klapotke group say this is one of the most sensitive materials they have handled, you better take notice.
Some choice comments from the paper:
The precipitated N10 compound 4 was not dried in the funnel
because attempts to manipulate the dry solid inevitably led to
extremely loud explosions and the destruction of labware.
we experienced several inadvertent explosions
during handling such as allowing the dry powder to slide down
the inside of a Raman tube or slowing down the rotation rate of a
rotary evaporator
Inadvertent explosions is a lovely expression.
They conclude that the formation of this compound leads to speculation that even longer nitrogen linkages might be possible but note that it “may present challenges for isolation”.
No kidding!