Following up my previous post, I went into the proposed legislation and found the relevant section, which I reproduce here:
SEC. 1152. ADDITION OF SYNTHETIC DRUGS TO SCHEDULE I OF THE CONTROLLED SUBSTANCES ACT.
(a) Cannabimimetic Agents- Schedule I, as set forth in section 202(c) of the Controlled Substances Act (21 U.S.C. 812(c)) is amended by adding at the end the following:
‘(d)(1) Unless specifically exempted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of cannabimimetic agents, or which contains their salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation.
‘(2) In paragraph (1):
‘(A) The term ‘cannabimimetic agents’ means any substance that is a cannabinoid receptor type 1 (CB1 receptor) agonist as demonstrated by binding studies and functional assays within any of the following structural classes:
‘(i) 2-(3-hydroxycyclohexyl)phenol with substitution at the 5-position of the phenolic ring by alkyl or alkenyl, whether or not substituted on the cyclohexyl ring to any extent.
‘(ii) 3-(1-naphthoyl)indole or 3-(1-naphthylmethane)indole by substitution at the nitrogen atom of the indole ring, whether or not further substituted on the indole ring to any extent, whether or not substituted on the naphthoyl or naphthyl ring to any extent.
‘(iii) 3-(1-naphthoyl)pyrrole by substitution at the nitrogen atom of the pyrrole ring, whether or not further substituted in the pyrrole ring to any extent, whether or not substituted on the naphthoyl ring to any extent.
‘(iv) 1-(1-naphthylmethylene)indene by substitution of the 3-position of the indene ring, whether or not further substituted in the indene ring to any extent, whether or not substituted on the naphthyl ring to any extent.
‘(v) 3-phenylacetylindole or 3-benzoylindole by substitution at the nitrogen atom of the indole ring, whether or not further substituted in the indole ring to any extent, whether or not substituted on the phenyl ring to any extent.
‘(B) Such term includes–
‘(i) 5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol (CP-47,497);
‘(ii) 5-(1,1-dimethyloctyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol (cannabicyclohexanol or CP-47,497 C8-homolog);
‘(iii) 1-pentyl-3-(1-naphthoyl)indole (JWH-018 and AM678);
‘(iv) 1-butyl-3-(1-naphthoyl)indole (JWH-073);
‘(v) 1-hexyl-3-(1-naphthoyl)indole (JWH-019);
‘(vi) 1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole (JWH-200);
‘(vii) 1-pentyl-3-(2-methoxyphenylacetyl)indole (JWH-250);
‘(viii) 1-pentyl-3-[1-(4-methoxynaphthoyl)]indole (JWH-081);
‘(ix) 1-pentyl-3-(4-methyl-1-naphthoyl)indole (JWH-122);
‘(x) 1-pentyl-3-(4-chloro-1-naphthoyl)indole (JWH-398);
‘(xi) 1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201);
‘(xii) 1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694);
‘(xiii) 1-pentyl-3-[(4-methoxy)-benzoyl]indole (SR-19 and RCS-4);
‘(xiv) 1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole (SR-18 and RCS-8); and
‘(xv) 1-pentyl-3-(2-chlorophenylacetyl)indole (JWH-203).’.
(b) Other Drugs- Schedule I of section 202(c) of the Controlled Substances Act (21 U.S.C. 812(c)) is amended in subsection (c) by adding at the end the following:
‘(18) 4-methylmethcathinone (Mephedrone).
‘(19) 3,4-methylenedioxypyrovalerone (MDPV).
‘(20) 2-(2,5-Dimethoxy-4-ethylphenyl)ethanamine (2C-E).
‘(21) 2-(2,5-Dimethoxy-4-methylphenyl)ethanamine (2C-D).
‘(22) 2-(4-Chloro-2,5-dimethoxyphenyl)ethanamine (2C-C).
‘(23) 2-(4-Iodo-2,5-dimethoxyphenyl)ethanamine (2C-I).
‘(24) 2-[4-(Ethylthio)-2,5-dimethoxyphenyl]ethanamine (2C-T-2).
‘(25) 2-[4-(Isopropylthio)-2,5-dimethoxyphenyl]ethanamine (2C-T-4).
‘(26) 2-(2,5-Dimethoxyphenyl)ethanamine (2C-H).
‘(27) 2-(2,5-Dimethoxy-4-nitro-phenyl)ethanamine (2C-N).
‘(28) 2-(2,5-Dimethoxy-4-(n)-propylphenyl)ethanamine (2C-P).’.
SEC. 1153. TEMPORARY SCHEDULING TO AVOID IMMINENT HAZARDS TO PUBLIC SAFETY EXPANSION.
Section 201(h)(2) of the Controlled Substances Act (21 U.S.C. 811(h)(2)) is amended–
(1) by striking ‘one year’ and inserting ‘2 years’; and
(2) by striking ‘six months’ and inserting ‘1 year’.
My first impression is that it reads like a patent. That is likely the best angle to take on this, something broad enough to cover what might be made but narrow enough to avoid over-reaching and impinging upon unrelated research work.
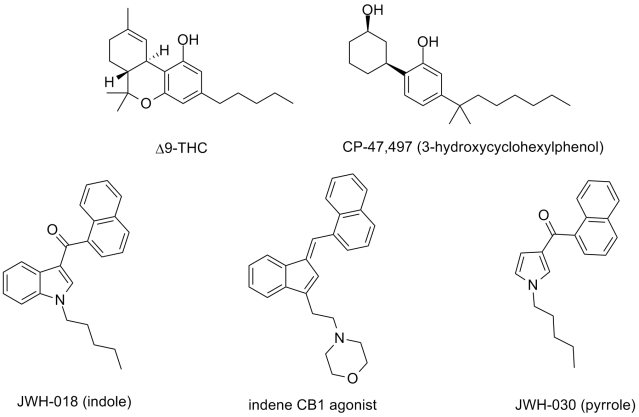
It looks like they covered the basic structural motifs seen in CP-47,497 (along with some related compounds like HU-243) and JWH-018, along with some related JWH indoles. I was glad to see that they were quite careful with the wording on the indoles, so that clear derivatives of the CB agonists would be covered but not necessarily all indole chemistry. They included the pyrroles and indenes but no specific examples of those, which I thought a weakness of the statement. The broad number of examples in the indoles leads me to think they are basing it on examples found in incense and bath salt products. I will also applaud the inclusion of a specific phrase that includes the measured biological activity (in binding and functional assays), which is to me at least the most defining feature of a CB agonist.
Reblogged this on Take A Look.
So under this act, in your opinion, would UR 144 and AB-001 be analogues of JWH-018? And would fluoro-UR-144 be an analogue of AM 2201? And how would you describe their relationship if you had to defend your opinion?
This was interesting. http://www.pennumbra.com/issues/pdfs/156-4/Kau.pdf
Thanks for the link, having a browse over that.
UR 144 has the tetramethylcyclopropane group where JWH-018 has a naphthyl. Reading over the definition from the legislation, it mentions naphthyl or naphthyl derived which would not cover the TMCP. And further it talks about CB1 activity whereas UR 144 is principally a CB2 agonist. AB-001 (with an adamantyl group) would also not be covered but that does seem to be active at CB1 (the adamantyl is supposed to confer CB2 selectivity but CB1 activity does remain). And this illustrates the difficulty is drawing up such legislation when the drug producers are also designing things to get around the law – they have a lot of chemical space to explore.
I guess I would also ask if (c4)-AB-001 and (c4) AB-001 would be analogues of JWH 073.
I’m asking where things that are not naphthoyl or naphthyl or any of the other classes that were banned fit in to this Act, being that otherwise, they are the same…they just have a 2,2,3,3 TMCP group or an adamantoyl group. Any opinion at all on this would be helpful. Thanks!
UR 144 has higher affinty for the CB2 receptors, yes. But that would be dose related. Although it prefers CB2, higher doses would cause both receptors to be bound. Because of that, it would be considered to have CB1 activity. Now, it can’t be called an analogue of any of the classes that were banned, but if it was compared, not to the class, but to individual rules that include specific chemical name, you could say that it looks like an analogue of JWH-018 with a substitution of TMCP where the Naphthoyl group was? Maybe? I’m looking at this in terms of actually being able to enforce this. How would you get consensus of opinion between experts? In the end, a judge decides I guess.
Reading the law like I would read a patent, I could make a very good case that naphthyl -> TMCP is not a trivial change, in other words if this was a patent and I wanted to make something similar but that was not covered, the TMCP would be a good analog to use if it showed the activity I wanted. The law covers naphthyl and benzoyl derivatives, but no med chemist would say they are highly related to aliphatic groups like cyclohexyl or cyclopropyl or adamantyl. And yes you are right, the CB1 activity is lower in UR144 but still a respectable Ki of 150 nM. Which was my latter point – it is very difficult to write a law that covers everything reasonable – depending on your view of reasonable here.
Excellent. Thank you! Very helpful…about what a med chemist would consider “similar” in respect to this class of chemicals.
In NM, our law does not distinguish between receptor binding so that part is not an issue as it is with limits of federal law. Here’s that section.
16.19.20.65 SCHEDULE I:
C(32) Synthetic cannabinoids: Unless specifically exempted or unless listed in another schedule, any material, compound, mixture of preparation which contains any quantity of the following synthetic cannabinoids which demonstrates binding activity to the cannabinoid receptor or analogs or homologs with binding activity:
(a) CP 55,244 ((hydroxymethyl)-4-[2-hydroxy-4-(2-methyloctan-2-yl)phenyl]
1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalen-2-ol)
(b) CP 55,940 (5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol)
(c) JWH-081 (1-pentyl-3-[1-(4-methoxynaphthoy)]indole)
(d) JWH-122 (1-pentyl-3-(4-methyl-1-naphthoyl)indole)
(e) JWH-133 3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro -6,6,9-trimethyl-6H
dibenzo[b,d]pyran
(f) JWH 203 1-pentyl-3-(2-chlorophenylacetyl)indole)
(g) JWH 210 4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone
(h) AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole)
(i) AM-1221 (1-(N-methylpiperdin-2-yl)methyl-2-methyl-3-(1-naphthoyl)-6-nitroindole
(j) AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole)
(k) RCS-4 or SR-19 (1-pentyl-3-[(4-methoxy)-benzoyl]indole)
(l) RCS-8 or SR-18 (1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole)
(m) JWH-210 (1-pentyl-3-(4-ethylnaphthoyl)indole)
(n) WIN-49,098 (Pravadoline) (4-methoxyphenyl)-[2-methyl-1-(2-morpholin-4-ylethyl)indol-
3-yl]methanone
(o) WIN-55,212-2 (2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-1,4-benzooxazin6-
yl)-1-naphthalenylmethanone)
(p) Any of the following synthetic cannabinoids, their salts, isomers, and salts of isomers, unless specifically excepted, whenever the existence of these salts, isomers, and salts of isomers is possible within the specific chemical designation:
(i) Naphthoylindoles: Any compound containing a 3-(1- naphthoyl) indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent including, but not limited to, JWH-015, JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-398 and AM-2201;
(ii) Naphthylmethylindoles: Any compound containing a1Hindol- 3-yl-(1-naphthyl) methane structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent including, but not limited to, JWH-175, JWH-184, and JWH-199;
(iii) Naphthoylpyrroles: Any compound containing a 3-(1- naphthoyl) pyrrole structure with substitution at the nitrogen atom of the pyrrole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent including, but not limited to, JWH-307;
(iv) Naphthylmethylindenes: Any compound containing a naphthylideneindene structure with substitution at the 3-position of the indene ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent including, but not limited to, JWH-176;
(v) Phenylacetylindoles: Any compound containing a 3- phenylacetylindole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent including, but not limited to, JWH-203, JWH-250, JWH-251, and RCS-8;
(vi) Cyclohexylphenols: Any compound containing a 2-(3- hydroxycyclohexyl) phenol structure with substitution at the 5- position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4-morpholinyl) ethyl group, whether or not substituted in the cyclohexyl ring to any extent including, but not limited to, Cannabicyclohexanol (CP 47,497 C8 homologue), CP 47,497 and CP 55,490
(vii) Benzoylindoles: Any compound containing a 3-(benzoyl) [ 5 ] OTS-3833.4 indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl) methyl, or 2-(4- morpholinyl) ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent including, but not limited to, AM-694, Pravadoline (WIN 48,098), RCS-4, and AM-1241;
I’m sorry for bombarding you, I just think this is a very interesting topic.
Here is another approach to dealing with this which I like a little better.
Click to access Shining-Star-enterprises-affirmation-with-exhibits.pdf
No problem, it is an interesting topic! And a good discussion.
This is another informative blurb about interpretation of that Act.
http://www.erowid.org/psychoactives/law/analog/analog_info1.shtml
I have another question for you about the analogue stuff. This company markets Ab-001 as a JWH 018 adamantyl analog. How would a med chemist defend this idea or more specifically, can it be defended reasonably?
http://www.caymanchem.com/app/template/Product.vm/catalog/9000799
I think that is a reasonable description. It is a short-hand way of describing something by comparing it to something that people are familiar with. All it means by ‘analog’ is “it is like JWH-018 except with an adamantyl group”. I think it OK and that they list further description below (‘adamantyl …demonstrate improved affinity and selectivity’) though they neglect to say selectivity for CB2 and not sure the reason for pointing out it is found in herbal incenses. Equally I doubt anyone is buying it from Cayman for illicit use far too expensive for one thing!
Ok. So, for ease of advertising, you would consider that an accurate description. But, in a court of law, you would make the case that it is not an analogue based on the difference in CB1/CB2 receptor affinities and the idea that the adamantyl group is a pretty big change. Correct?
Basically, any chemical structure that someone wishes to prosecute under the federal analogue act has to be presented in a court of law, case by case and based on the information provided, the judge will decide. (I keep picturing dueling med chemists and others on the witness stands…he said, she said type stuff). In addition, even if the judge in one case decides that a substance would fall under the Act, the substance does not become specifically banned…and another court can decide the opposite in another case. There’s no consistancy. It’s all based on who gives the best argument for or against in court. Nothing is set in stone and deciding if these things have medical value is being left up to the courts rather than the medical/scientific community. I believe this is a dangerous road we are going down and the area of medical research is going to be hit the hardest IMO. I have no problem with the courts going after the manufacturers and distributers for things like fraud, mislabeling, misbranding, “mail” crimes etc., but I’m not sure about this whole analogue thing. We have too many laws out there meant to go after criminals but the innocent get hurt in the process. I guess that’s the problem with zeal without knowledge?
(apologies for not replying to your previous comment, combination of a v. busy week in the lab and a trip out of town)
Up until now, the standard has been the same chemical structure. That’s pretty clear cut, but limited when similar structures can have similar activity – especially as the designer drug spice/K2 got going. So the law is trying to be a little more proactive and anticipate what might show up in the next batch of bath salts. But as you rightly say, the trouble is “what is the same?” My reading of this law limits this quite a lot – it needs a naphthyl type group on the indole for example. They allow that others things might be hung off the indole or naphthyl but require that basic structure. Which is why I would say the adamantyl would not be covered by this law.
Of course a further problem might be what different judges say about it. How that might play out I cannot say.
This really is going to be a test of how well this Act works or doesn’t work. I guess we shall see how far the definition gets stretched. You know, if wellbutrin wasn’t already classified as a dangerous drug, RX only…and it was found in bath salts…we’d slap a cathinone label on it, and throw it in the trash…in otherwords, it would be a missed opportunity.
Paracetamol is a cannabimimetic. It is also Acetaminophen. Congrats to the govt for banning ibuprofen. We’re all very proud of the politiciatns we sent for 10 years to a quality chemistry program to be able to police my body. Thanks. That’s sarcasm by the way. They banned pain relievers. Good job. Way to go. Lets ban first and test later…wait, no I meant never. None of the Schedule 1 drugs has ever been dropped from that listing even though there are 50 years of study overseas showing half of that list is dubious at best and the supporting evidence for the initial bans was spurious at worst, but downright criminal in most instances. Hearst and the propaganda machine in the 30s comes to mind.
How do i mix these effectively, to produce a legal high?
how does this affect drug development research using the “usual suspect” cannabinoids e.g. cbg, cbn, cbc, & cetera
Would THC acetate be a cannabimimetc? And is that prodrug?
An answer would be great, if you have the time.
Sorry for the delay in response, this blog is not really a going concern at the moment.
THC acetate absolutely is a cannabimimetic and also a pro-drug, I am sure that acetyl drops off in the body and that is a very common way to make pro-drugs of difficult to absorb agents.
Thank you very much. And thanks for this site, too. Have a great day.